Abstract
Background:
Even though children with acute myeloid leukemia (AML) receive a very intensive chemotherapy and most achieve a complete remission (CR) ~30% of patients suffer from relapse. Post-treatment monitoring of measurable residual disease (MRD) can allow detection of a re-emerging leukemic clone several months before clinical relapse, and studies are in progress that aim at treating children with a molecular relapse.
Specific genetic aberrations can be used for disease monitoring after therapy completion, but even though oncogenic fusion transcripts are more common in childhood than adult AML, NPM1 mutation is much rarer and consequently MRD measurements based on these aberrations are clinically applicable in only ~40% of childhood AML patients. Thus, a considerable fraction of patients do not have a suitable leukemia-specific molecular MRD target, but genes with an abnormally high expression in the leukemic cells might be candidate MRD targets in those patients. WT1 overexpression in childhood AML is well described, and if distinctly overexpressed at diagnosis, serves as a suitable MRD target in a large proportion of patients.
Gene expression profiling has identified several other genes with an abnormally high expression in the leukemic blasts compared to normal hematopoietic cells.
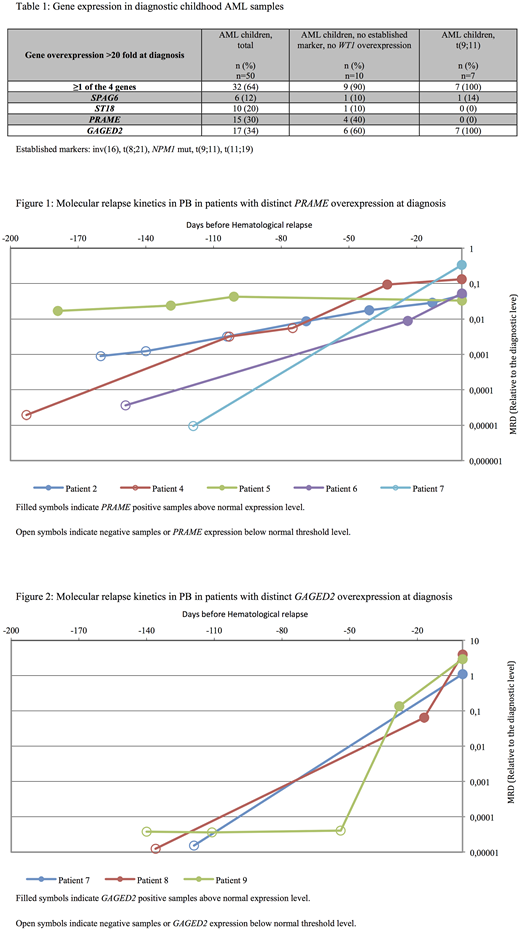
We investigated the applicability of 4 leukemia-associated genes (PRAME, GAGED2, ST18, SPAG6) as targets for early detection of relapse in peripheral blood (PB) in a Danish cohort of childhood AML patients, defined child-specific reference values of gene expression based on a large material of PB and BM samples from hematologically healthy children, and investigated gene expression levels under the presence of infection.
Methods:
We investigated the expression of 4 leukemia-associated genes (PRAME, GAGED2, ST18, SPAG6) in hematologically healthy children (n=53) and during suspected infection in febrile but otherwise healthy children (n=90). Gene expression in de novo AML at diagnosis (n=50) and during follow-up (n=20) was compared with child-specific reference values. We defined the 95th percentile of expression levels in hematologically healthy children as the upper limit of normal expression.
RT-qPCR analyses were performed in compliance with EAC protocols and due to concordant qPCR efficiencies the ΔΔCq method for relative quantification could be applied.
Results:
At AML diagnosis, 64% had high expression of at least 1 of the 4 genes defined as >20-fold overexpression compared to hematologically healthy children. Nine out of 10 patients (90%) without established molecular MRD targets or high WT1 expression had high expression of at least 1 of the 4 genes. All 7 children with t(9;11) had GAGED2>1000-fold overexpressed. Gene expression was quantified in 99 PB samples (163 RT-qPCR analyses) during follow-up in 20 patients with distinct overexpression at diagnosis. All 10 patients with PB sampling performed within 100 days of disease recurrence displayed expression above normal by a median of 1.6 months (range 0.5-6 months) before hematological relapse. Patients with CBF-AML had a significantly longer interval between molecular relapse and hematological relapse than patients with non-CBF-AML (2.5 months (range 0.8-6 months) vs. 0.9 months (range 0.5-2 months), p=0.047). One patient with PB sampling performed only once at 119 days prior to hematological relapse did not show any molecular evidence of disease recurrence before hematological relapse. Only 1 of 96 (1%) post-therapy follow-up analyses performed in 9 patients in continuous CR for >5 years after diagnosis had expression above normal. In this case, a 9-year-old girl in continuous CR had an increase in ST18 expression, however the increase was transient and returned to normal level in the following samples.
We found no clinically relevant influence of fever on gene expression levels, except for GAGED2, where 21% of febrile children had expression above normal.
Conclusions:
Sequential post-therapy monitoring of overexpressed genes in PB can predict relapse in childhood AML patients and facilitates molecular MRD monitoring in 90% of patients without a leukemia-specific target or WT1 overexpression. Frequent PB sampling (every 4-6 weeks) is necessary to detect an upcoming relapse, however in the post-treatment follow-up setting PB serves as an attractive and easily accessible source of preference compared to BM aspiration.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal